The amount of heat Q absorbed by a substance is related to its increase of temperature

by the following relationship:
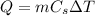
where m is the mass of the substance and
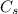
is its specific heat.
Using

,

and

, we can find the specific heat of the substance by re-arranging the formula:

and looking at the table of specific heat values for various substance, we find that this value corresponds to
lead.