The boiling point of water can be calculated by the equation:
Where:
P = Pressure in mm Hg
Po = Atmospheric pressure in mm Hg
ΔH= heat of vaporization in kJ/mol
R = Ideal Gas Constant (J/mol-K)
To = normal boiling point in Kelvin
T = boiling point of water (K)
Our known values are:
P = 630 mm Hg
Po = 760 mm Hg
ΔH = 40.66 kJ/mol = 40.66×1000 =40660
R = 8.314 J mol⁻¹ K ⁻¹
To = 373 K
Putting these values in the equation,
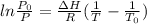
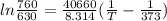
Solving the equation will give:
T=370K
so, the boiling point of water is 370 K.