Answer: The correct answer is Option D.
Step-by-step explanation:
Calcium is the 20th element of the periodic table having electronic configuration
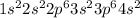
This element will loose 2 electrons easily to form stable configuration. This is a metal and will form an ionic bond with a non-metal.
From the given options:
Option A: boron, aluminum, gallium; 3
These elements belong to Group 13 and are considered as metals having oxidation state of +3 because they easily loose 3 electrons. Thus, they will not form any compound with calcium.
Option B: sulfur, selenium, polonium; 6
These elements belong to Group 16 and are considered as non-metals having oxidation state of -2 because they easily gain 2 electrons. Thus, they form compound with calcium but in a 1 : 1 ratio.
Option C: oxygen, tellurium, bismuth; 6
These elements belong to Group 16 and are considered as non-metals having oxidation state of -2 because they easily gain 2 electrons. Thus, they form compound with calcium but in a 1 : 1 ratio.
Option D: nitrogen, phosphorus, arsenic; 5
These elements belong to Group 15 and are considered as non-metals having oxidation state of -3 because they easily gain 3 electrons. Thus, they form compound with calcium in a 3 : 2 ratio.
Hence, the correct answer is Option D.