Answer:
For 1: The number of atoms of zinc are
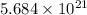 atoms.
atoms.
For 2: Mass of zinc reacted are 95.1279 grams.
Step-by-step explanation:
For the given chemical reaction, the equation follows:
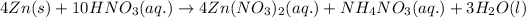
To calculate the number of moles, we use the equation:
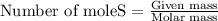 ....(1)
....(1)
For nitric acid:
Mass of nitric acid = 1.49 grams.
Molar mass of nitric acid = 63 g/mol
Putting values in equation 1, we get:
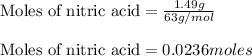
By Stoichiometry of the reaction:
10 moles of nitric acid reacts with 4 moles of zinc metal.
So 0.0236 moles of nitric acid will react with =
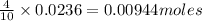 of zinc metal
of zinc metal
Now, according to Mole concept:
1 mole of an element contains
 number of atoms.
number of atoms.
So, 0.00944 moles of zinc will contain
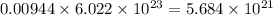 number of atoms.
number of atoms.
Hence, the number of atoms of zinc are
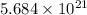 atoms.
atoms.
For ammonium nitrate:
Given mass of ammonium nitrate = 29.1 grams
Molar mass of ammonium nitrate = 80 g/mol
Putting values in equation 1, we get:
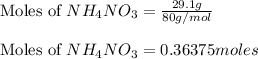
By Stoichiometry of the given reaction:
1 mole of ammonium nitrate is produced from 4 moles of zinc.
So, 0.36375 moles of ammonium nitrate will be produced from =
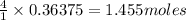 of zinc.
of zinc.
Now, calculating the mass of zinc.
Moles of zinc = 1.455 moles
Molar mass of zinc = 65.38g/mol
Putting values in equation 1, we get:
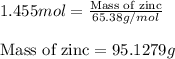
Hence, mass of zinc reacted are 95.1279 grams.