Answer:
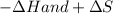 will favor a spontaneous change
will favor a spontaneous change
Explanation:
For a spontaneous reaction,
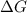 (Change in gibbs free enegy) should be negative.
(Change in gibbs free enegy) should be negative.
We know,
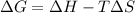
where
 is change in enthalpy for reaction, T is temperature in kelvin scale and
is change in enthalpy for reaction, T is temperature in kelvin scale and
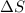 is change in entropy for reaction.
is change in entropy for reaction.
T is always positive. Therefore , to make
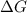 negative,
negative,
 should be negative and/or
should be negative and/or
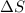 should be positive
should be positive
Hence
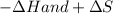 will favor a spontaneous change.
will favor a spontaneous change.