Answer: Mass of water present in 250 g of solution is 165 g
Step-by-step explanation:
A solution contains solute and solvent. A solute is defined as the substance which is present in smaller proportion and solvent is defined as the substance which is present in larger proportion.
We are given:
34 % (m/m) of LiCl
This means that 34 g of LiCl is present in 100 g of solution.
Mass of water in solution = 100 - 34 = 66 g
We need to find the mass of water present in 250 g of solution. By applying unitary method, we get:
In 100 g of solution, mass of water present in 66 g
So, in 250 g of solution, mass of water present will be =
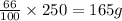
Hence, mass of water present in 250 g of solution is 165 g