Step-by-step explanation:
An ionic compound is the compound formed due to transfer of electrons from one atom to another.
Atomic number of calcium is 20 with electronic distribution 2, 8, 8, 2. So, on losing 2 valence electrons it forms
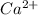 ion.
ion.
Atomic number of chlorine is 17 with electronic configuration 2, 8, 7. So, on gaining one electron it forms
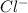 .
.
As, a calcium ion has +2 charge and a chlorine ion has -1 charge.
Therefore, when both of them chemically combine together then two chlorine ions are needed to combine with one calcium atom.
As a result,
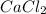 is formed.
is formed.