Answer : The correct option is, (D)
Explanation :
Option A reaction :
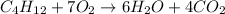
It is a combustion reaction. A reaction in which a hydrocarbon react with the oxygen to give product as carbon dioxide and water.
Option B reaction :
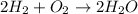
It is a combination reaction. A reaction in which the two or more reactants react to give a product.
Option C reaction :
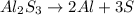
It is a decomposition reaction. A reaction in which a reactant decomposes to form two or more products.
Option D reaction :
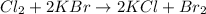
It is a single displacement reaction. It is a reaction in which the more reactive element displace the less reactive element. In this reaction, most reactive element chlorine displaces the less reactive element bromine.
Hence, the correct option is, (D)