Answer: Formation of precipitate indicates that there has been a chemical reaction.
Step-by-step explanation:
Chemical reaction: It is defined as change in arrangement of the atoms so as to form a new substance.
Mixing of two clear solutions that is lead nitrate and potassium iodide gives out yellow solid precipitate of lead iodide with aqueous solution of potassium nitrate.
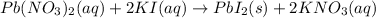
The physical evidence which indicates that chemical reaction has been taken place is formation of yellow solid precipitate of lead iodide ,(
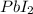 ).
).