Answer: Lanthanide and actinide series
Explanation: The elements with partially or fully filled f orbitals belong to f block elements.
f block elements include lanthanides and actinides. They are called so the lanthanides contain the first element named as lanthanum and actinides contain first element named as actinium. They are also called as inner transition elements.
All of them have general electronic configuration:
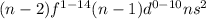 , where n = 6-7
, where n = 6-7