Answer:

Explanation:
a)
 : This is a non polar covalent compound which are held by weak vanderwaal forces of attraction.
: This is a non polar covalent compound which are held by weak vanderwaal forces of attraction.
b)
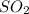 : This is a covalent compound which is polar due to the presence of lone pair of electrons and are held by dipole-dipole forces of attraction.
: This is a covalent compound which is polar due to the presence of lone pair of electrons and are held by dipole-dipole forces of attraction.
c)
 : These are joined by a special type of dipole dipole attraction called as hydrogen bond. It forms between electronegative nitrogen atom and hydrogen atom and is the strongest interaction.
: These are joined by a special type of dipole dipole attraction called as hydrogen bond. It forms between electronegative nitrogen atom and hydrogen atom and is the strongest interaction.
d)
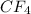 : This is a covalent compound and is non polar which are held by weak vanderwaal forces of attraction.
: This is a covalent compound and is non polar which are held by weak vanderwaal forces of attraction.
e)
 : This is a covalent compound and is non polar which are held by weak vander waal forces of attraction.
: This is a covalent compound and is non polar which are held by weak vander waal forces of attraction.