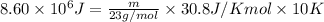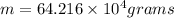Answer:
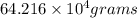 of liquid sodium is required.
of liquid sodium is required.
Explanation: To calculate the mass of liquid sodium, we use the formula:
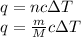
where,
q = heat required,
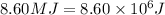 (Conversion factor: 1MJ = 1000000J)
(Conversion factor: 1MJ = 1000000J)
m = Mass of liquid sodium,
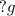
M = Molar mass of liquid sodium,
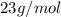
c = Specific heat capacity,
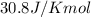
 = change in temperature,
= change in temperature,
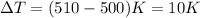 (Conversion factor: 0°C = 273K)
(Conversion factor: 0°C = 273K)
Putting values in above equation, we get: