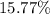Answer : The percent of aluminium in
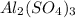 is
is
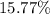
Solution :
Molar mass of Aluminium = 26.98 g/mole
Molar mass of
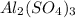 = 342.15 g/mole
= 342.15 g/mole
First we have to calculate the mass of Aluminium.
Mass of 2 Al atoms = 2 × 26.98 g/mole = 53.96 g/mole
Now we have to calculate the percent composition of Aluminium in
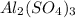
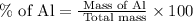
Now put all the given values in this formula, we get
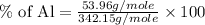
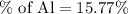
Therefore, the percent of aluminium in
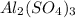 is
is