Answer: The average kinetic energy of the molecules will increase.
Step-by-step explanation: We are given a container having fixed volume of 5.00L and there is an increase in the temperature of the cotainer from 20°C to 250°C.
The average kinetic energy is directly related to the temperature of the gas.
Mathematically,
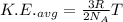
From the above expression, as we increase the temperature of the gas, the average kinetic energy of the gas increases and vice-versa.
In the given question, the temperature of the gas is increasing and hence, the average kinetic energy of the molecules will also increase.