mass number is defined as sum of number of protons and neutrons
so here for Isotope A
Isotope A contains 56 protons and 80 neutrons.
Mass number will be given as
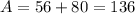
For Isotope B
Isotope B contains 55 protons and 81 neutrons.
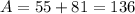
For Isotope C
Isotope C contains 57 protons and 80 neutrons.
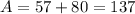
For Isotope D
Isotope D contains 56 protons and 74 neutrons.
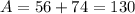
So here Isotope A and Isotope B has same mass number