1. Nuclear equations
Answer:
D.
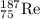
Explanation:
Your unbalanced nuclear equation is
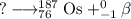
It is convenient to replace the question mark by an atomic symbol,
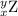 , where x = the atomic number, y = the mass number, and Z = the symbol of the element .
, where x = the atomic number, y = the mass number, and Z = the symbol of the element .
Then your equation becomes
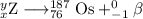
The main point to remember in balancing nuclear equations is that the sums of the superscripts and the subscripts must be the same on each side of the equation.
Then
x = 76 - 1 = 75
y = 187 + 0 = 187
Element 75 is rhenium (Re), so the nuclear equation becomes
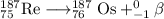
===============
2. Nuclear Particles
Answer:
a. a beta particle and a positron
Explanation:
A beta particle is a negative electron (
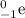 ) and a positron is a positive electron (
) and a positron is a positive electron (
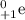 ).
).
Thus, both have the same mass but opposite charge.
b. is wrong. A neutron and a proton have the same mass number but slightly different masses. Furthermore, a neutron has no charge, while a proton has a positive charge.
c. is wrong. A proton and an electron and an electron have opposite charges, but a proton has 1836 times the mass of an electron.
d. is wrong. An alpha particle has four times the mass and twice the charge of a proton .