Answer : The pressure of the gas would be unchanged.
Explanation : Given,
Ideal gas law :
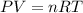
where,
P = pressure of gas
V = volume of gas
T = temperature of gas
n = moles of gas
R = Gas constant
The expression for pressure is,
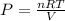
when the volume and temperature of gas doubled then the expression for pressure is,
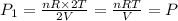
From this we conclude that the
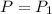 that means pressure of the gas would be unchanged.
that means pressure of the gas would be unchanged.