Answer: The chemical reaction is written below.
Step-by-step explanation:
Double displacement reaction is defined as the reaction in which exchange of ions takes place.
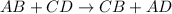
When an aqueous solution of calcium chloride reacts with aqueous solution of potassium carbonate, it produces solid solution of calcium carbonate and aqueous solution of potassium chloride.
The chemical equation for the reaction follows:
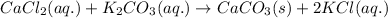
Hence, the chemical reaction is written above.