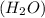Answer: 10.86 moles
Explanation: For the given chemical reaction. 2 moles of lead combines with 2 moles of lead oxide and 2 moles of sulphuric acid to produce 2 moles of lead sulphate and 2 moles of water.
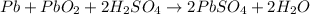
1 mole of lead oxide
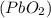 produces 2 moles of water
produces 2 moles of water
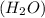
Thus 5.43 moles of lead oxide
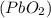 produces =
produces =
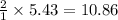 moles of water
moles of water