Answer : The initial quantity of sodium metal used is 17.25 grams.
Solution : Given,
Volume of
 gas = 8.40 L
gas = 8.40 L
Molar mass of Na metal = 23 g/mole
The Net balanced chemical reaction is,
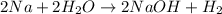
At STP, 22.4 L of volume is occupied by 1 mole of
 gas
gas
so, 8.40 L of volume is occupied by =
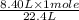 = 0.375 moles of
= 0.375 moles of
 gas
gas
Now from the above reaction, we conclude that
1 mole of
 gas produced by the 2 moles of Na metal
gas produced by the 2 moles of Na metal
0.375 moles of
 gas produced =
gas produced =
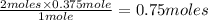 of Na metal
of Na metal
The quantity of Na metal used = Moles of Na metal × Molar mass of Na metal = 0.75 moles × 23 g/mole = 17.25 grams
Therefore, the initial quantity of sodium metal used is 17.25 grams.