The relationship is r = 2.5Z + 130.
From your graph, I estimate the slope of the line to be:
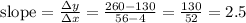
The y-intercept ≈ 130.
So, the equation for the line is
r = 2.5Z + 130.
The relation tells us that the atomic size increases as the atomic number increases.
This makes sense because, as you go down a Group, you are adding electrons to the next shell further out.