Answer:
1) Volume of water displaced = 5.16 L
2) Copper sinks in water.
Step-by-step explanation:
1) Density of copper = 8.92
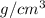
Mass of copper = 46 kg = 46000 g
We know that, Density = Mass / volume
So, Volume = Mass/ density
Volume of copper = 46000/8.92 = 5156.95
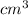
Since the density of copper 8.92
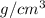 is greater than density of water 1
is greater than density of water 1
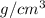 , the copper immerses in water. Since it immerses it will displace a volume of water which is equal to the volume of copper.
, the copper immerses in water. Since it immerses it will displace a volume of water which is equal to the volume of copper.
So volume of water displaced = volume of copper = 5156.95
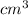 =5.16 L of water
=5.16 L of water
2) Since the density of copper 8.92
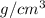 is greater than density of water 1
is greater than density of water 1
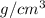 , the copper immerses in water. So copper sink in water.
, the copper immerses in water. So copper sink in water.