Number of moles is defined as the ratio of given mass in g to the molar mass.
The mathematical formula is:
Number of moles =
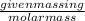 (1)
(1)
Number of zinc atoms is equal to
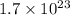 , by Avogadro number, number of moles can be calculated.
, by Avogadro number, number of moles can be calculated.
As, 1 mol=
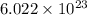 atoms, hence,
atoms, hence,
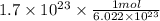
= 0.2822 mol
Now, from formula (1), calculate mass in g (molar mass of zinc = 65.4 g/mol)
0.2822 mol =
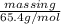
mass in g =
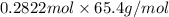
= 18.45588 g
Thus, by rounding off the above number, it will come near about 19 g approximately.
Hence, option (C) is the correct answer.