Answer:
58.3%
Step-by-step explanation:
The formula of the compound is given as:
CsPO₄
Now, the percentage composition of Cs in the compound is = ?
So;
To find this parameter, find the molar mass of the compound:
Molar mass of CsPO₄ = 132.9 + 31 + 4(16) = 227.9g/mol
Percentage composition of Cs=
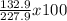 = 58.3%
= 58.3%