Answer:
63.6%
Step-by-step explanation:
The given compound is:
N₂O;
The problem here is to find the percent composition of nitrogen in the compound.
First find the molar mass of the compound:
Molar mass of N₂O = 2(14) + 16 = 44g/mol
So;
Percentage composition of Nitrogen =
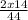 x 100 = 63.6%
x 100 = 63.6%