Answer:
Oxygen receives two electrons from magnesium.
Step-by-step explanation:
Hello!
In this case, considering the chemical reaction by which magnesium oxide is produced:
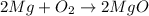
In that case, if we show up the oxidation states, we have:
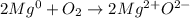
In such a way, since metals, like magnesium, have the capacity to lose electrons, rather than receive them, we infer why it turns out with +2 rather than -2; thus, we the correct answer is "oxygen receives two electrons from magnesium." because it results with -2 in MgO.
Best regards!