Answer:
The minimum frequency required to ionize the photon is 111.31 ×
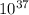 Hertz
Hertz
Given:
Energy = 378
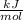
To find:
Minimum frequency of light required to ionize magnesium = ?
Formula used:
The energy of photon of light is given by,
E = h v
Where E = Energy of magnesium
h = planks constant
v = minimum frequency of photon
Solution:
The energy of photon of light is given by,
E = h v
Where E = Energy of magnesium
h = planks constant
v = minimum frequency of photon
738 ×
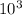 = 6.63 ×
= 6.63 ×
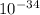 × v
× v
v = 111.31 ×
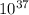 Hertz
Hertz
The minimum frequency required to ionize the photon is 111.31 ×
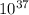 Hertz
Hertz