the initial condition is given as -
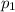 =33,000 pa
=33,000 pa
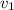 =8.0 litre i.e 8×
=8.0 litre i.e 8×
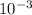
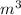
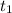 =300k
=300k
final state is given as-
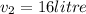 i.e16
i.e16
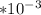
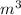
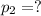
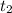 =300k[the temperature is constant here]
=300k[the temperature is constant here]
here the temperature is constant.we have to calculate the final pressure
as per the Boyle's law,
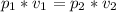
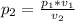
=
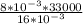
=16500 pa [ans]