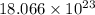Answer : The number of barium ion is,
Explanation :
The formula of barium phosphate is,
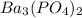
Barium phosphate is formed from three barium ion and 2 phosphate ion by criss-cross method. That means,
1 mole of barium phosphate dissociates to give 3 moles of barium ion and 2 moles of phosphate ion.
Or,
As we know that, 1 mole contains
 number of ions.
number of ions.
1 mole of barium phosphate dissociates to give
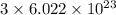 moles of barium ion and
moles of barium ion and
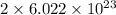 moles of phosphate ion.
moles of phosphate ion.
The number of barium ion =
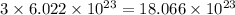
The number of phosphate ion =
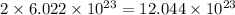
Hence, the number of barium ion is,