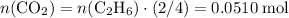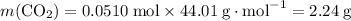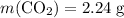
Ethene react with oxygen at a
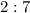 molar ratio:
molar ratio:
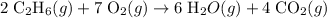
Convert the quantity of each reactant supplied to number of moles of particles:
The question stated not whether both reactants were used up in this process. Thus start by testing the assumption that e.g., ethene was used up while some oxygen gas were left unreacted (ethene as the limiting reagent.) Under this assumption, the relative availability of the two species,
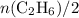 and
and
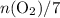 (as seen in the balanced chemical equation) shall satisfy the relationship
(as seen in the balanced chemical equation) shall satisfy the relationship
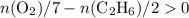
In other words,
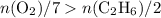
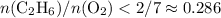
Evaluating the expression
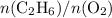 with data given in the question yields approximately
with data given in the question yields approximately
 , which does satisfy the relationship. Hence the assumption holds and ethene is the limiting reactant.
, which does satisfy the relationship. Hence the assumption holds and ethene is the limiting reactant.
The quantity of a reactant produced in a chemical reaction is related to its stoichiometric (of relating to proportions) relationship with the limiting reactant (or any of the reactants in case of more than one limiting reactant.) For this scenario, given the molar ratio

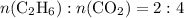 ,
,