The relationship between change in Gibb's free energy, change in enthalpy and change in entropy is:
Δ
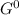 = Δ
= Δ
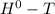 Δ
Δ
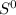
For a reaction at equilibrium, Δ
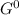 = 0
= 0
So Δ
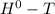 Δ
Δ
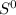 = 0
= 0
Δ
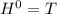 Δ
Δ
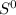
-92 kJ/mol = T(-0.199kJ/(mol.K))
T = 462 K
At temperatures greater than 462 K, the value T [/tex]Δ
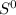 would become more than Δ
would become more than Δ
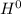 , giving a negative value for Δ
, giving a negative value for Δ
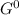 . Therefore, the reaction would become spontaneous at temperatures > 462 K
. Therefore, the reaction would become spontaneous at temperatures > 462 K