Answer:- 0.81 moles of ions.
Solution:- The dissociation equation for the given compound is as follows...
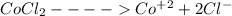
From this, 1 mol of cobalt(II)chloride gives 1 mol of cobalt ion and 2 moles of chloride ion. So, 1 mol of the compound gives total 3 moles of the ions.
Now, we can calculate the moles of ions produced by 0.27 moles of the compound as...
0.27 mol of compound x (3 moles of ions/1 mol of compound) = 0.81 moles of ions