Answer: The correct answer is decreased by two.
Step-by-step explanation:
Alpha particle is emitted when a radioactive isotope undergoes alpha decay process. In this process a heavier particle decays into a lighter particle with the release of alpha particle. The alpha particle carries a mass of 4 units and a charge of +2 units.
The atomic number of the atom gets reduced by 2 units and atomic mass of the same atom gets reduced by 4 units.
The equation representing alpha decay process is:
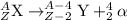
Hence, the correct answer is decreased by two.