m = mass of sample = 15.6 g = 0.0156 kg
c = specific heat of the sample = 0.58 kcal/kgC = 580 cal/(kgC)
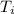 = initial temperature = 21.5 C
= initial temperature = 21.5 C
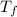 = final temperature = ?
= final temperature = ?
Q = heat absorbed = 0.207 kcal = 207 cal
using the equation
Q = m c (
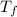 -
-
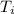 )
)
207 = (0.0156) (580) (
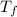 -21.5)
-21.5)
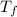 = 44.4
= 44.4