Answer:
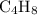 .
.
Step-by-step explanation:
Look up the relative atomic mass of
 ,
,
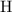 , and
, and
 on a modern periodic table:
on a modern periodic table:
Calculate the molecular mass of
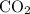 and
and
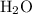 :
:
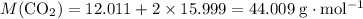 ;
;
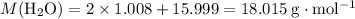 .
.
Given the mass
 of
of
 and
and
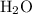 produced, calculate the number of moles of molecules that were produced:
produced, calculate the number of moles of molecules that were produced:
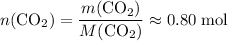 ;
;
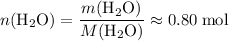 .
.
Calculate the number of moles of
 atoms and
atoms and
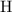 atoms in these
atoms in these
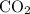 and
and
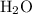 molecules.
molecules.
- Each
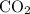 molecule contains one
molecule contains one
 atom. Therefore, that
atom. Therefore, that
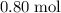 of
of
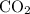 contains
contains
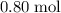 of
of
 atoms.
atoms. - Each
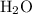 molecule contains two
molecule contains two
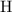 atoms. Therefore, that
atoms. Therefore, that
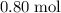 of
of
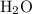 contains
contains
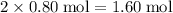 of
of
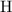 atoms.
atoms.
The combustion reaction here include two reactants: the hydrocarbon and
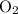 .
.
As the name suggests, hydrocarbons contain only
 atoms and
atoms and
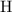 atoms. On the other hand,
atoms. On the other hand,
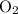 contains only
contains only
 atoms.
atoms.
Therefore, all the
 and
and
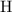 atoms in those
atoms in those
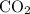 and
and
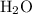 molecules are from the unknown hydrocarbon. (With a similar logic, all the
molecules are from the unknown hydrocarbon. (With a similar logic, all the
 atoms in those combustion products are from
atoms in those combustion products are from
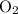 .)
.)
In other words, that
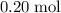 of this unknown hydrocarbon molecules contains:
of this unknown hydrocarbon molecules contains:
Hence, each of these hydrocarbon molecules would contain
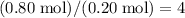 carbon atoms and
carbon atoms and
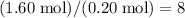 hydrogen atoms.
hydrogen atoms.
The molecular formula of this hydrocarbon would be
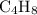 .
.