Hello!
Data:
P (pressure) = 1 atm
V (volume) = 18.5 L
T (temperature) = 300 K
n (number of mols) = ? (in mol)
R (Gas constant) = 0.082 (atm*L/mol*K)
Apply the data to the Clapeyron equation (ideal gas equation), see:
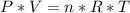
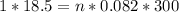
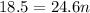
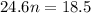
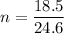
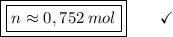
Note:
If the feedback is to be considered, the closest r
esponse is 0.751 mol Nacl
_________________
_________________
I hope this helps. =)