This question above is based on Dalton's Law of Partial Pressure. This law states that "the total pressure of a system of gas is equal to the sum of the pressure of each individual gas (partial pressure).
the Partial Pressure of a gas = (mole fraction) × (total pressure of the system)
⇒ Partial Pressure of Hydrogen =
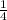
×
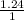
= 0.31 atm
Thus the Partial Pressure of Hydrogen in the container is
0.31 atm.