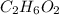Answer:
B)
Step-by-step explanation:
First you should add up the three percentages:
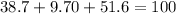
Then you take that base of 100g to calculate the mass of each element:
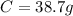
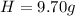
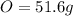
Then you find the number of moles of each element using the molar mass of each one:
- For C:
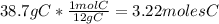
- For H:
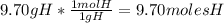
- For O:
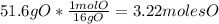
Then, you find the minimum number of moles and divide each one by it:
- For C:
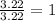
- For H:
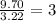
- For O:
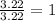
You have find the proportion of each element in the compound that is
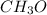 , but you should find the molecular formula taking in account the molar mass of the compound, so:
, but you should find the molecular formula taking in account the molar mass of the compound, so:
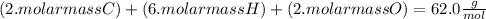
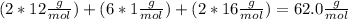
So the molecular formula is