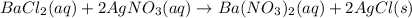Answer: Double replacement reactions
Explanation: In general, a double replacement reaction is written as:
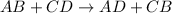
This type of reactions takes place if an insoluble product(precipitate) is formed which could be predicted using solubility table or rules.
For example, chloride of silver ion is insoluble. So, if we mix barium chloride and silver nitrate solutions then the double replacement reaction takes place and a precipitate of silver chloride(AgCl) is formed.