we can use the combined gas law equation to find the new pressure of the gas.
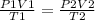
where P - pressure
V - volume
T - temperature
parameters for the first instance are on the left side and parameters for the second instance are on the right side of the equation
P1 - 795 mm Hg x 0.0013 atm/ mm Hg = 1.033 atm
T1 - 23.5 °C + 273 = 296.5 K
T2 - 31.7 °C + 273 = 304.7 K
substituting the values in the equation
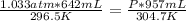
P = 0.712 atm
the answer closest to this value is A) 0.723 atm
therefore answer is
A) 0.723 atm