Answer : The percent composition of P = 43.63 % and O = 53.37 % .
Empirical formula of Phosphorous oxide = P₂O₅
Part A : Percent composition :
It is percent of each element present in compound . It is given by formula :
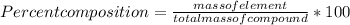
Given : Mass of Phosphorous (P ) = 30.98 g
Mass of Compound Phosphorous oxide = 71.00 g
Mass of Oxygen (O) = mass of compound - mass of P
= 71.00 g - 30.98 g = 40.02 g
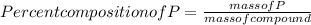
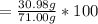
Percent composition of P = 43.63 %
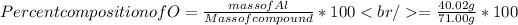
Percent composition of O = 53.37 %
Part B : Empirical formula of Phosphorous Oxide.
Empirical formula is formula which shows the proportion of element present in a compound . Following are the steps to calculate empirical formula of an compound :
Step 1 : Find masses of each element .
Mass of P = 30.98 g
Mass of O = 40.02 g
Step 2 : Conversion of masses of element to its mole .
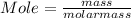
Given : Mass of P = 30.98 g
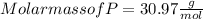
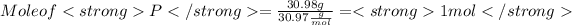
Given: Mass of O = 40.02 g
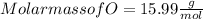
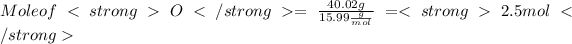
Step 3 : Finding Ratio of mole .
In this step ratio is found by dividing each mole by smallest mole .Since mole of P is smaller , so this will be used for division.
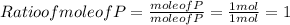
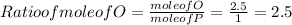
Hence , Ratio of P : O = 1 : 2.5
Since the ratio is in fraction , it need to be converted to whole number . So we multiply the ratio by such a minimum number which gives us a whole number ratio. This step is skipped if the ratio already comes in whole number.
On multiplication the ratio by 2 :
Ratio of P : O = ( 1 : 2.5 ) * 2 = 1 * 2 : 2.5 * 2
Ratio of P : O = 2 : 5
Step 4 : Writing the empirical formula
The ratio of P and O is 2: 5 , which gives the empirical formula of compound as P₂O₅ .