Question:
For the cell constructed from the hydrogen electrode and metal-insoluble salt electrode, B) calculate the mean activity coefficient for 0.124 b HCl solution if E=0.342 V at 298 K
Answer:
The mean activity coefficient for HCl solution is 0.78.
Step-by-step explanation:
Activity coefficient is defined as the ratio of any chemical activity of any substance with its molar concentration. So in an electrochemical cell, we can write activity coefficient as γ. The equation for determining the mean activity coefficient is
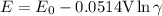
As we know that
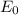 = 0.22 V and E = 0.342 V, the equation will become
= 0.22 V and E = 0.342 V, the equation will become
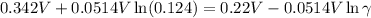

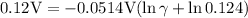

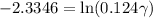
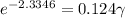

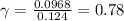
So, the mean activity coefficient is 0.78.