Answer:
They are found in various parts of the periodic table: the whole group of the noble gases (8A), as well as some elements of alkali metals (1A), groups 5A, 6A and 7A (halogens)
Step-by-step explanation:
There are a total of 11 elements in the periodic table which are gases at room temperature and standard atmospheric pressure.
Considering the periodic table, there's first of all one prominent group, the noble gases, group 8A. The whole group contains gases, those are helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe) and radon (Rn).
The remaining ones are also typically diatomic at room temperature. First of all, hydrogen (H) in group 1A. At room temperature, it exists as a diatomic gas,
 .
.
We also have one diatomic gas in group 5A, this is nitrogen,
 . Another diatomic gas is found in group 6A, this is oxygen,
. Another diatomic gas is found in group 6A, this is oxygen,
 .
.
The final gases belong to the group of halogens, group 7A, those are also diatomic, fluorine (
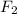 ) and chlorine (
) and chlorine (
 ).
).