Answer:
There are 5 moles of the element Clarkanium in 190 gm.
Step-by-step explanation:
The atomic mass of the element Clarkanium is 38 grams.
Given mass of the element = 190 gm
The mole of a substance is the mass of the substance containing same number of atoms that is in 12-grams of carbon-12.
So the atomic mass of the element is equal to the mass of 1 mole of the element.
Therefore number of moles can be calculated by dividing given mass of the element by atomic mass of the element.
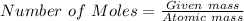
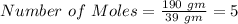
Since Moles is calculated in number, so it has no unit.
Hence there are 5 moles of the element Clarkanium in 190 gm.