Answer:
T = 525K
Step-by-step explanation:
The temperature of the two-level system can be calculated using the equation of Boltzmann distribution:
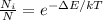 (1)
(1)
where Ni: is the number of particles in the state i, N: is the total number of particles, ΔE: is the energy separation between the two levels, k: is the Boltzmann constant, and T: is the temperature of the system
The energy between the two levels (ΔE) is:
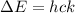
where h: is the Planck constant, c: is the speed of light and k: is the wavenumber
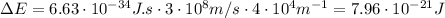
Solving the equation (1) for T:
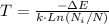
With Ni = N/3 and k = 1.38x10⁻²³ J/K, the temperature of the two-level system is:
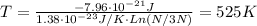
I hope it helps you!