Answer: 21.7 torr
Step-by-step explanation:
As the relative lowering of vapor pressure is directly proportional to the amount of dissolved solute.
The formula for relative lowering of vapor pressure will be,
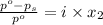
where,
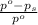 = relative lowering in vapor pressure
= relative lowering in vapor pressure
i = Van'T Hoff factor = 1 (for non electrolytes)
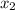 = mole fraction of solute =
= mole fraction of solute =
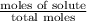
Given : 32.7 g of glycerol is present in 100 g of aqueous solution, thus (100-32.7) g = 67.3 g of water
moles of solute (glycerol) =
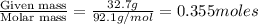
moles of solvent (water) =
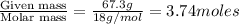
Total moles = moles of solute (glycerol) + moles of solvent (water) = 0.355 + 3.74 = 4.095
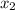 = mole fraction of solute =
= mole fraction of solute =
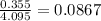
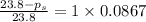
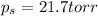
Thus the vapor pressure of the solution at
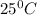 is 21.7 torr
is 21.7 torr