Answer: Molarity of the given hydrochloric acid solution is 0.106 M.
Step-by-step explanation:
Given:
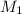 = 0.143 M,
= 0.143 M,
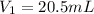
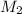 = ? ,
= ? ,
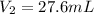
Hence, molarity of the given hydrochloric acid solution is as follows.
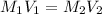
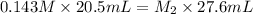
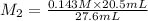
= 0.106 M
Therefore, molarity of the given hydrochloric acid solution is 0.106 M.