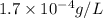Answer :
The solubility equilibrium reaction will be:
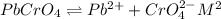
The solubility in grams per liter in water at
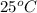 is,
is,
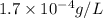
Explanation :
The solubility equilibrium reaction will be:
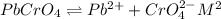
Let the solubility be 's'.
The expression for solubility constant for this reaction will be,
![K_(sp)=[Pb^(2+)][CrO_4^(2-)]](https://img.qammunity.org/2020/formulas/chemistry/college/ls75y9r4zx4fv47oemfugpk0xhez9ml96d.png)
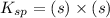
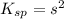
Given:
Solubility constant =
 =
=
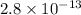
Now put all the given values in the above expression, we get:
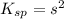
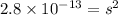
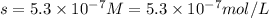
Now we have to convert the solubility in gram per liter.
Solubility =
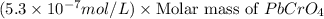
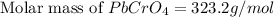
Solubility =
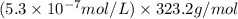
Solubility =
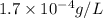
Therefore, the solubility in grams per liter in water at
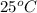 is,
is,