Answer : The volume of water and alcohol present in 675 mL of this solution are 405 mL and 270 mL respectively.
Explanation :
As we are given that 40.0 % (v/v) alcohol solution. That means, 40.0 mL of alcohol present 100 mL of solution.
Now we have to calculable the volume of alcohol in 675 mL solution.
As, 100 mL of solution contains 40.0 mL of alcohol
So, 675 mL of solution contains
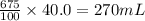 of alcohol
of alcohol
Thus, the volume of alcohol = 270 mL
Now we have to calculate the volume of water.
Volume of water = Volume of solution - Volume of alcohol
Volume of water = 675 mL - 270 mL
Volume of water = 405 mL
Thus, the volume of water = 405 mL
Hence, the volume of water and alcohol present in 675 mL of this solution are 405 mL and 270 mL respectively.