Answer:
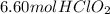
Step-by-step explanation:
Hello!
In this case, since the molecular formula of the chlorous acid is HClO2, we can see that is atomic mass is 68.46 g/mol, we can set up the following dimensional analysis to obtain the number of moles:
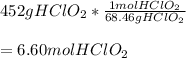
Ad the grams of chlorous acid are cancelled out.
Best regards!